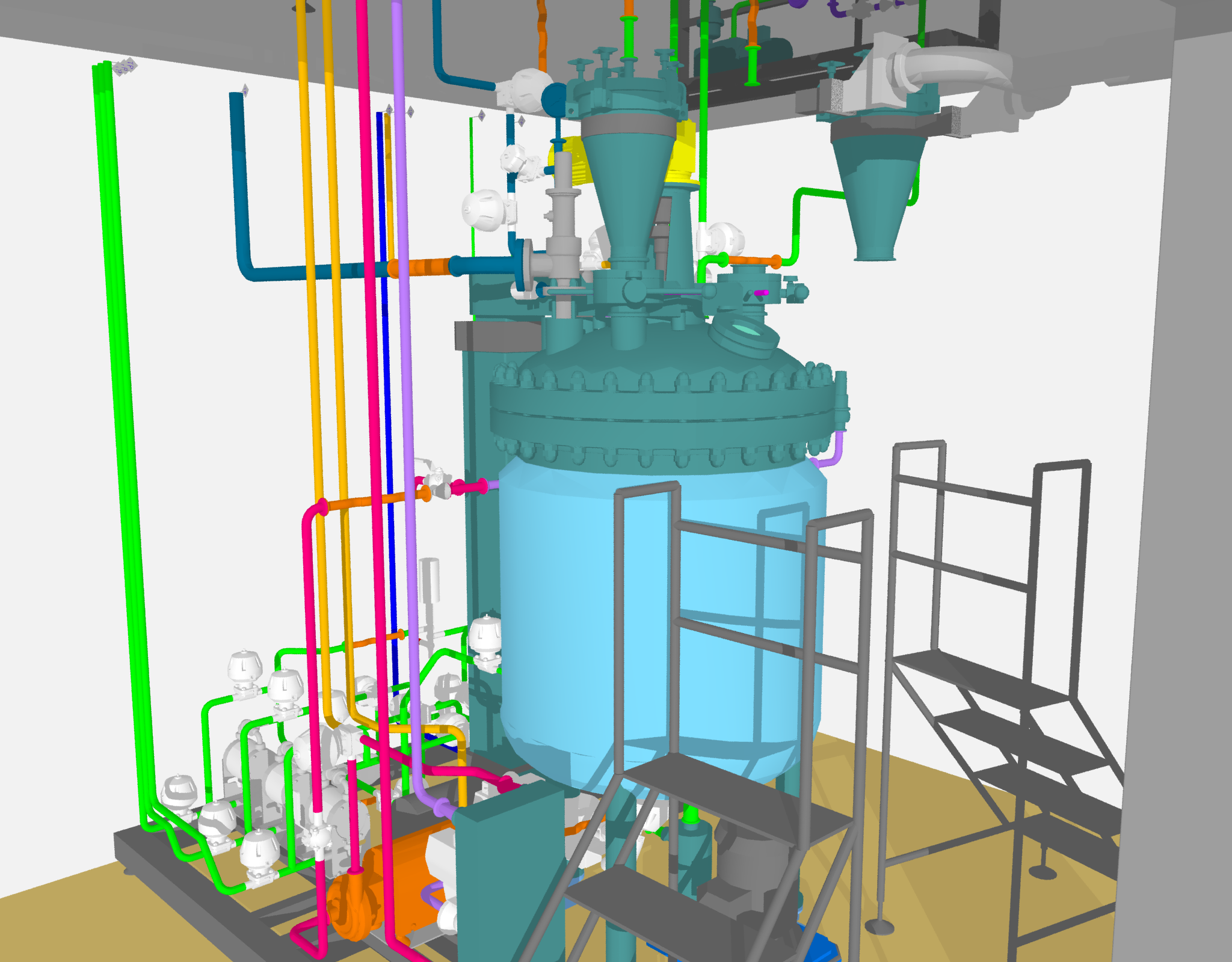
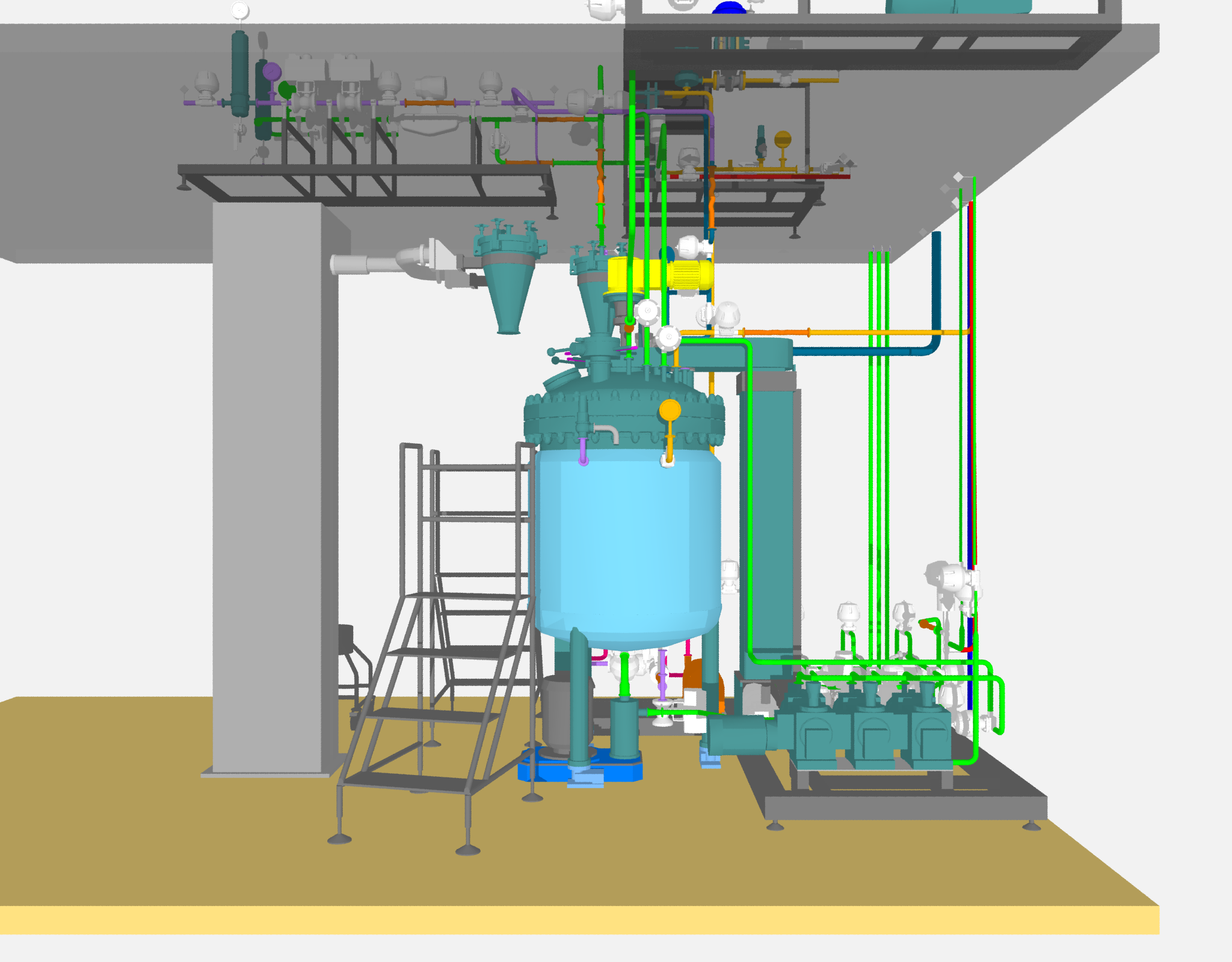
Pressurised Metered-Dose Inhaler (pMDI) Production Systems
Briggs of Burton have been involved designing and building pressurised Metered-Dose Inhaler (pMDI) production systems. Briggs of Burton have provided a number of these systems to several major Pharmaceutical companies offering inhalers to relieve the symptoms of asthma and Chronic Obstructive Pulmonary Disease (COPD). Many of these projects used the bronchodilator Salbutamol as the Active Pharmaceutical Ingredient (API) that is suspended in a HFA-134a or HFA 227 based formulation, which would be transferred and filled into pressurised Metered Dose Inhalers (pMDI).
Several of these systems were built under Briggs of Burton’s Giusti brand and included the high pressure suspension preparation vessel, Drug Addition Vessel (DAV) and lift system, the HFA mixing vessels and supply pipework and overall control system.
Pressurised Metered-Dose Inhaler (pMDI) Production Equipment
- Active Pharmaceutical Ingredient (API) Drug Addition Vessel (DAV)
- Containment and introduction of API to suspension vessel via DAV
- Skid-mounted Suspension Preparation Vessel
- Mixing and homogenisation solutions
- High material finish (electro-polish)
- High pressure, sanitary vessel and pipework (10 bar)
- Zero-deadleg valves (3rd Party)
- Utilities and service supply
- HFA 134a and HFA 227 conditioning tanks and supply
- Automation and control
- Fully validated system
- Integration into high-level site control system
- Installation and commissioning in clean room environment
- Pharmaceutical validation and commissioning (DQ/IQ/OQ/PQ)
pMDI System and HFA Tank References
Briggs of Burton have provided p-MDI Systems to a number of clients on projects around the world including: USA, UK, Pakistan, France, Spain, Germany. A summary of our experience is listed below:
- Metered-Dose Inhaler (MDI) System ranging from 50 to 800 Litre capacity
- HFA 134a and HFA 227 Mixing Vessel ranging from 26 to 750 Litre capacity
- Drug Addition Vessels (DAV) ranging from 5 to 25 Litre capacity